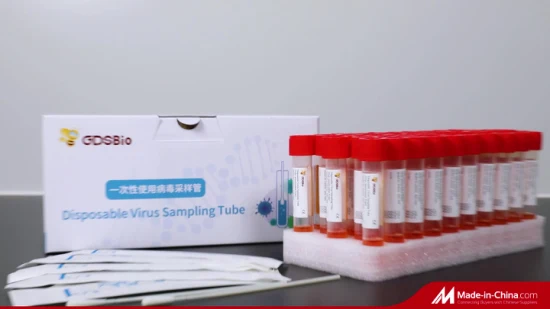
FDA CE Approved DNA Rna Test Kit Inactivated Inactivation Nasal Transport Medium Vtm Disposable Specimen Collection Virus Sampling Tube
GDSBio provides various types of sampling tubes Product Name Disposable Virus Sampling Tube (Inactivation Type) Packing
Description
Basic Info
| Model NO. | ST04 |
| Transport Package | Carton Packaging |
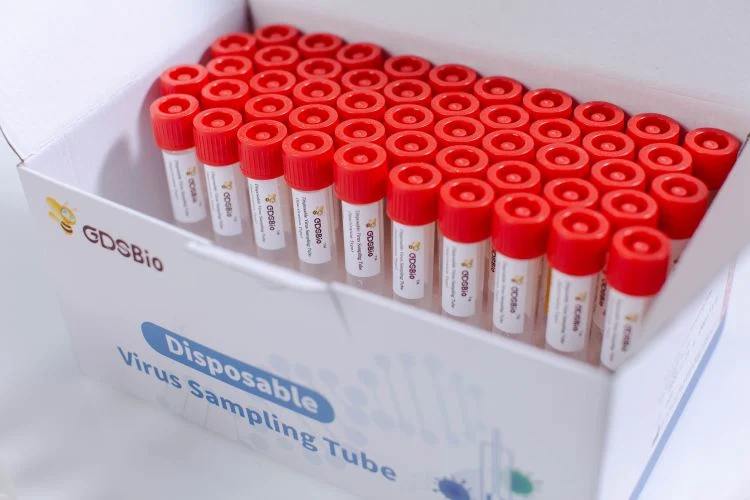
| Specification | 50 pieces/box |
| Trademark | GDSBio |
| Origin | China |
| HS Code | 3822001000 |
| Production Capacity | 500000/Week |
Product Description
GDSBio provides various types of sampling tubes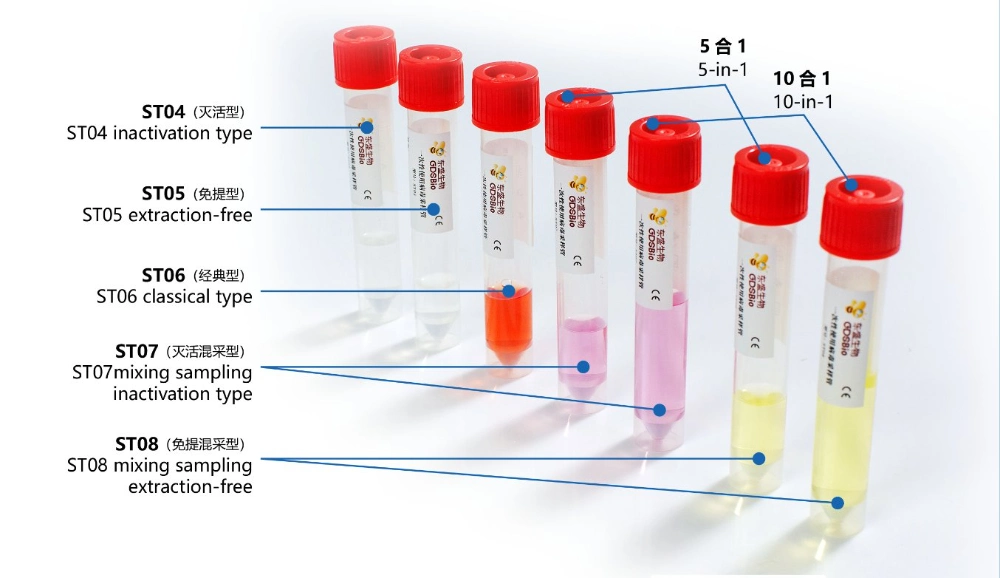
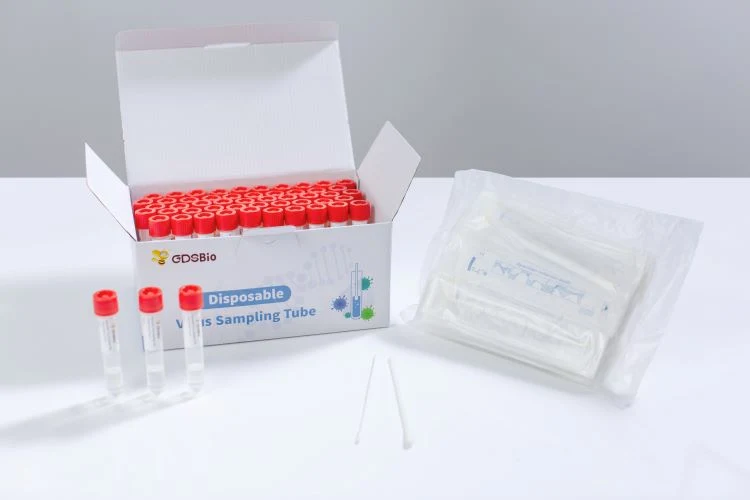
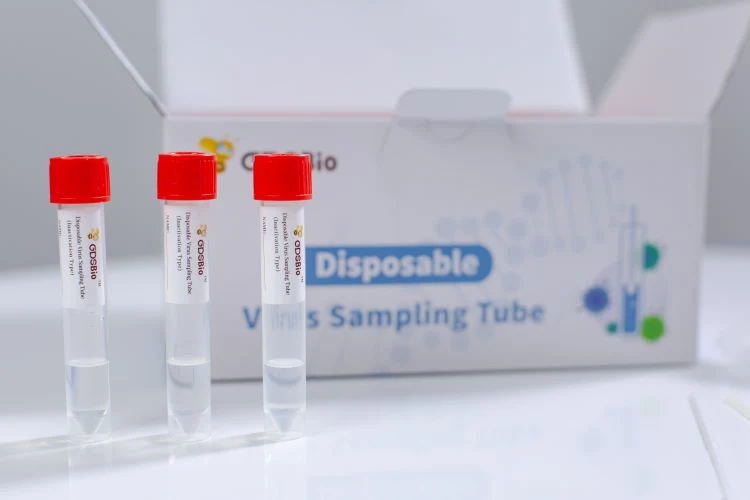
Disposable Virus Sampling Tube (Inactivation Type)
Packing Components
| Cat. No. | Specification of Tube | Volume of Preservation Solution | Package Size |
| F4001a | 2 ml | 1 ml | 1 pc/bag; 50 pcs/box |
| F4002a | 5 ml | 2 ml | 1 pc/bag; 50 pcs/box |
| F4003a | 10 ml | 3 ml | 1 pc/bag; 50 pcs/box |
| Cat. No. | Specification of Tube | Volume of Preservation Solution | Package Size |
| F4001b | 2 ml | 1 ml | 1 set/bag; 50 sets/box |
| F4002b | 5 ml | 2 ml | 1 set/bag; 50 sets/box |
| F4003b | 10 ml | 3 ml | 1 set/bag; 50 sets/box |
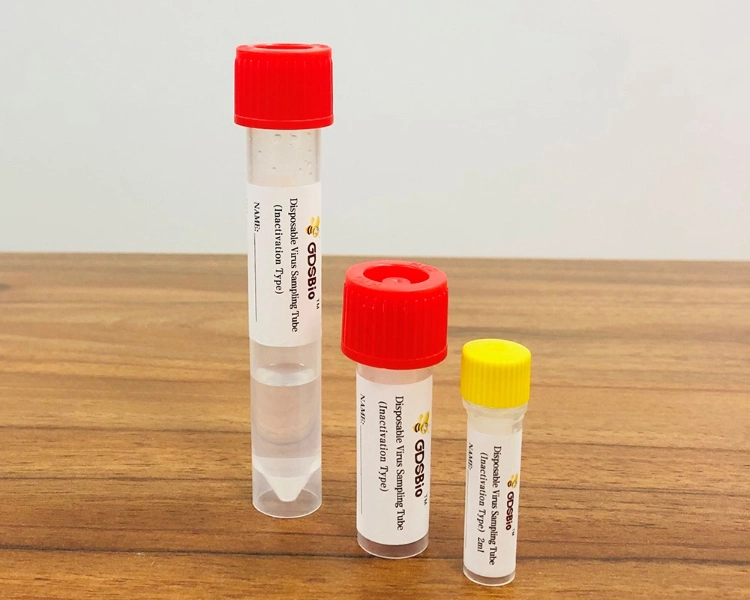
Disposable Virus Sampling Tube (Inactivation Type) is used for the collection, transportation and storage of virus specimens.
Storage Conditions and Shelf Life
Stored at 5 ~ 25°C, with a validity period of 12 months.
Safe/High Nucleic Acid Yield/Room Temperature Stable
Certification: CE/ US FDA/ China FDA
Certificate for Exportation
Nylon-flocked ABS stick nasal swab, throat/oral swab Environmentally friendly and strong carton packing
Prev: Labfil 25mm PVDF Hydrophilic HPLC Syringe Filter 0.45um Pre-Filter Welded Type
Next: Sample Prepared 25mm Ca Welded Syringe Filter 0.22um or 0.45um with Printing for Laboratory
Our Contact
Send now